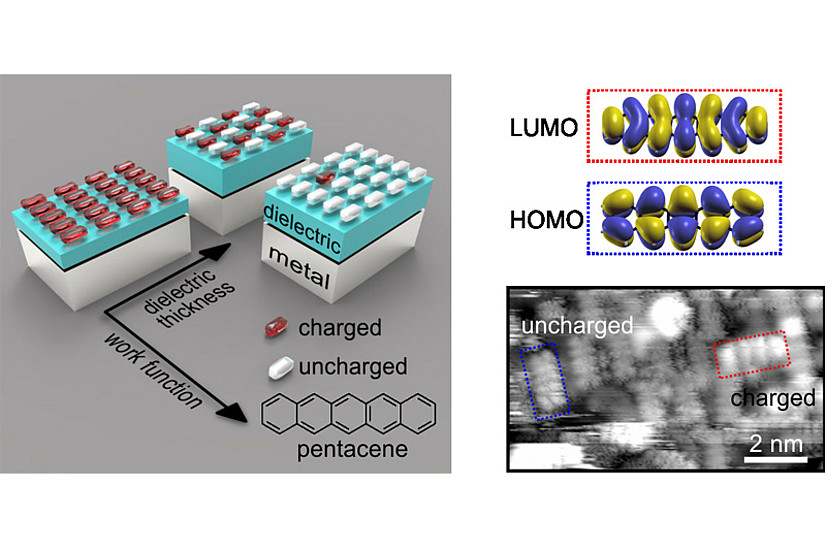
Ultrathin dielectric layers can be found in many technologies ranging from catalysis to organic electronics. Their presence (whether intentional or unintentional) can have profound influence on the chemical and electronic properties of a material. For example, it has been shown that ultrathin dielectrics on metals can reduce the work function of the metal such that charge transfer via tunneling through the dielectric layer into adsorbed atoms or molecules becomes possible, which might have strong effect on the surface reactivity, magnetic moments and charge injection at the contacts of organic devices. Whether this charge transfer occurs in integer units of electronic charge or not, and how the potential equilibration at the interface is accomplished, has long been a matter of debate and remained elusive from an experimental point of view.
In a recent publication (link: https://onlinelibrary.wiley.com/doi/full/10.1002/admi.202000592) the team around Michael G. Ramsey, Martin Sterrer and Peter Puschnig from the Institute of Physics could demonstrate with a combined experimental and computational work on a model system (ultrathin MgO films on Ag(100) with adsorbed pentacene molecules) that the charge transfer process and the potential equilibration is governed by the proportion of integer charged and uncharged molecules. Moreover, they could show that the ratio of charged and uncharged molecules can be controlled by either tuning the work function of the substrate or the thickness of the dielectric, confirming that the process can be understood and explained with a simple capacitor model. The understanding gained in this work demonstrates how charge transfer in such systems is controlled and paves the way for tuning their chemical and electronic properties in desired directions.