Electron orbitals show where and how electrons move around atomic nuclei and molecules. In modern chemistry and physics, they have proven to be a useful model for the quantum mechanical description and prediction of chemical bonding. Only if the orbitals match in space and energy can they be combined thereby forming a chemical bond. Michael Ramsey and Peter Puschnig from the Institute of Physics together with researchers from the Forschungszentrum Jülich have now shown that also the orbital’s momentum distribution is important to understand the bonding between organic molecules and metallic surfaces. The results were published in Nature Communications: https://doi.org/10.1038/s41467-022-32643-z and have been featured on a highlight webpage of the journal’s editors: https://www.nature.com/collections/wtpqpqpgwd
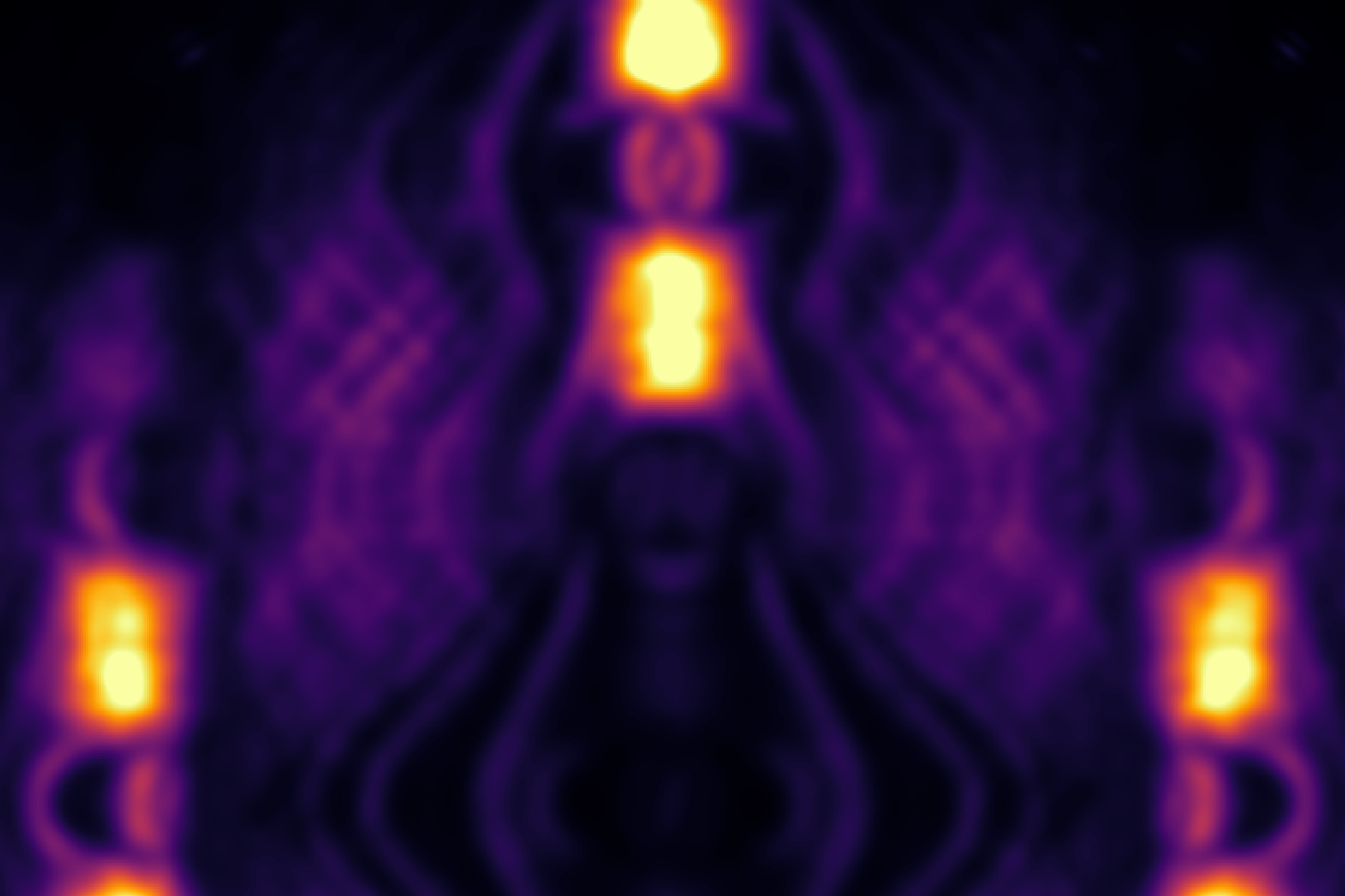
Chemical reactions are ultimately nothing more than the formation and breakdown of electron bonds, which can also be described as orbitals. Using the photoemission orbital tomography method, developed at the Institute of Physics at the University of Graz, the momentum distribution of the orbitals can also be mapped out experimentally. To this end, a monolayer of the organic molecule para-sexiphenyl, a blue-light emitting organic semiconducting molecule, adsorbed on a copper surface has been investigated at the Elettra Synchrotron in Trieste, Italy. Using synchrotron radiation and a so-called NanoESCA electron spectroscope, the angular distribution of the photoelectrons has been measured. The data reveals peculiar modifications in the molecular orbital’s momentum pattern upon adsorption on the metallic surface (see Figure).
To explain these momentum signatures in the hybrid organic/metal interface state, quantum mechanical ab-initio simulations within the framework of density functional theory have been performed. Taking into account the entire interacting system - molecules and metal surface, the experimental observations could not only be reproduced but also a novel selection criterion has been discovered. It demands that also the momenta of the metal and organic electrons must match for the hybrid bond to be formed.
Original publication:
Momentum-selective orbital hybridisation
Xiaosheng Yang, Matteo Jugovac, Giovanni Zamborlini, Vitaliy Feyer, Georg Koller, Peter Puschnig, Serguei Soubatch, Michael G. Ramsey, F. Stefan TautzNature Communications (Sept., 2022): https://doi.org/10.1038/s41467-022-32643-z