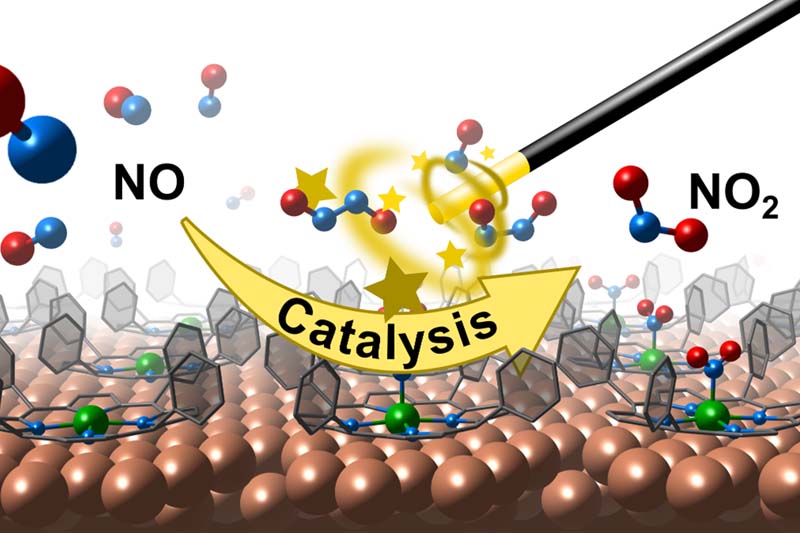
Catalysts are in general substances that increase the rate of a chemical reaction. Enzymes are nature’s most famous contribution to this subject, accelerating metabolic processes, enabling life itself. As nature is still unbeaten in its inventiveness, scientists often try to copy and utilize its ideas, a process known as biomimetics. The use of natural enzymes is, however, limited by several factors, giving birth to the wish of only using their reactive centre. Simple extraction of the chemically active site, on the other hand, faces the challenging problem to sustain the reactivity of the molecule, which is often imprinted in the whole enzyme.
A collaborative project with teams from Germany, Italy and the University of Graz has set to tackle this problem by combining biology and surface science. Their results were recently published in the journal Angewandte Chemie. A key constituent of several enzymatic reaction centres are (transition) metal containing tetrapyrrole molecules. Using a comprehensive range of experimental and theoretical methods, the study explores the reactivity of a nickel containing porphyrin complex toward nitric oxide (NO). Here, the metal centre of the molecule is activated by immobilization of the molecule on a copper surface leading to an uncommon and reactive metal oxidation state.
The interpretations of such measurements are far from being straightforward and further complicated by the high reactivity of different nitrogen oxides species and possible reaction intermediates. With the help of ab-initio quantum mechanical simulations, the work describes in detail the challenging complexity of the system and contributes the next step towards biomimetic single-atom site catalysts within the framework of surface science.
Original publication: Stredansky et al. Angew. Chem. Int. Ed. (2022) http://doi.org/10.1002/anie.202201916