Porphyrins and metalloporphyrins are versatile molecules, which offer a large variability of their properties due to the possibility to attach different ligands or metal atoms to the porphyrin macrocycle. The most important naturally occurring representatives of this molecule class are chlorophyll and hemoglobin.
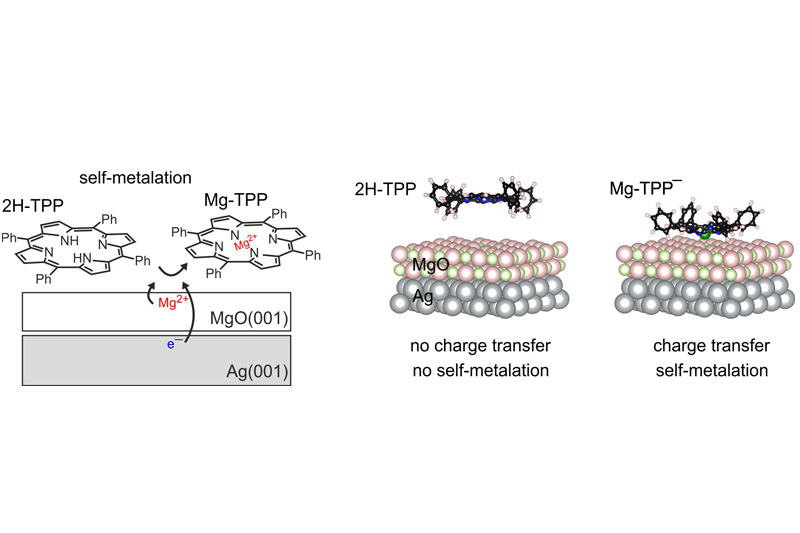
The surface-confined self-metalation reaction describes the replacement of two aminic protons in the macrocycle of the porphyrin molecule by a metal atom of the substrate. This reaction was first observed on metal surfaces, but more recent investigations show that it also occurs on oxides such as magnesium oxide (MgO). Previous work has shown that the self-metalation of 2H-tetraphenyl porphyrin on MgO is strongly dependent on the morphology of the material and occurs only on surface irregularities but not on a perfect planar surface.
A recently published collaborative work by the Surface Science group (Martin Sterrer, Mike Ramsey) and the Electronic Structure Theory group (Peter Puschnig) shows that the self-metalation reaction on MgO can also be induced on planar MgO surfaces. The key to this is the use of only few atomic layers thin MgO films, which are grown on a metallic substrate (silver). Because of the reduction of the work function of the silver substrate by the MgO film, electron transfer occurs into adsorbed 2H-TPP molecules, which then leads to the self-metalation reaction. By chemical tuning of the work function the researchers could furthermore show that states where the molecules are charged and metalated or uncharged and non-metalated can deliberately be generated. These results suggest a method to control the electric and chemical properties of porphyrins on surfaces, which opens the way for selective surface functionalization.
The work appeared in the journal Angewandte Chemie International Edition
L. Egger et al., Angew. Chem. Int. Ed., 2021, doi.org/10.1002/anie.202015187